Age, sex, and gonadal hormones differently influence anxiety- and depression-related behavior during puberty in mice
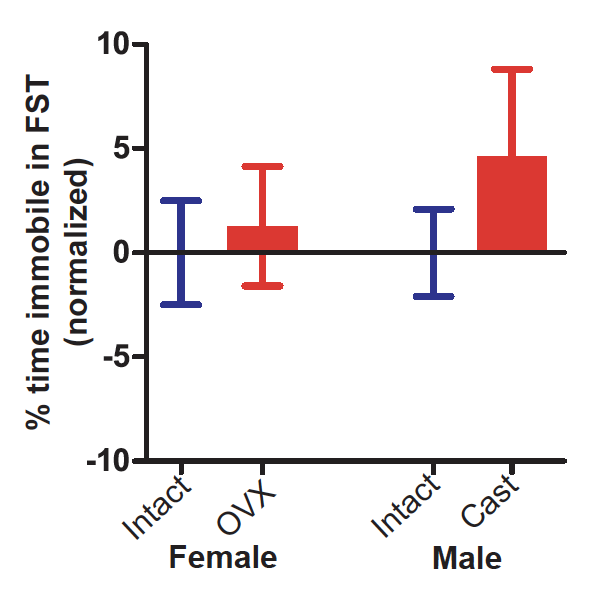
Anxiety and depression symptoms increase dramatically during adolescence, with girls showing a steeper increase than boys after puberty onset. The timing of the onset of this sex bias led us to hypothesize that ovarian hormones contribute to depression and anxiety during puberty. In humans, it is difficult to disentangle direct effects of gonadal hormones from social and environmental factors that interact with pubertal development to influence mental health. To test the role of gonadal hormones in anxiety- and depression-related behavior during puberty, we manipulated gonadal hormones in mice while controlling social and environmental factors. Similar to humans, we find that mice show an increase in depression-related behavior from pre-pubertal to late-pubertal ages, but this increase is not dependent on gonadal hormones and does not differ between sexes. Anxiety-related behavior, however, is more complex at puberty, with differences that depend on sex, age, behavioral test, and hormonal status. Briefly, males castrated before puberty show greater anxiety-related behavior during late puberty compared to intact males, while pubertal females are unaffected by ovariectomy or hormone injections in all assays except the marble burying test. Despite this sex-specific effect of pubertal hormones on anxiety-related behavior, we find no sex differences in intact young adults, suggesting that males and females use separate mechanisms to converge on a similar behavioral phenotype. Our results are consistent with anxiolytic effects of testicular hormones during puberty in males but are not consistent with a causal role for ovarian hormones in increasing anxiety- and depression-related behavior during puberty in females.
Josiah R. Boivin, David J. Piekarski, Jessica K. Wahlberg, Linda Wilbrecht, Age, sex, and gonadal hormones differently influence anxiety- and depression-related behavior during puberty in mice, Psychoneuroendocrinology (available online 12 August 2017), https://doi.org/10.1016/j.psyneuen.2017.08.009