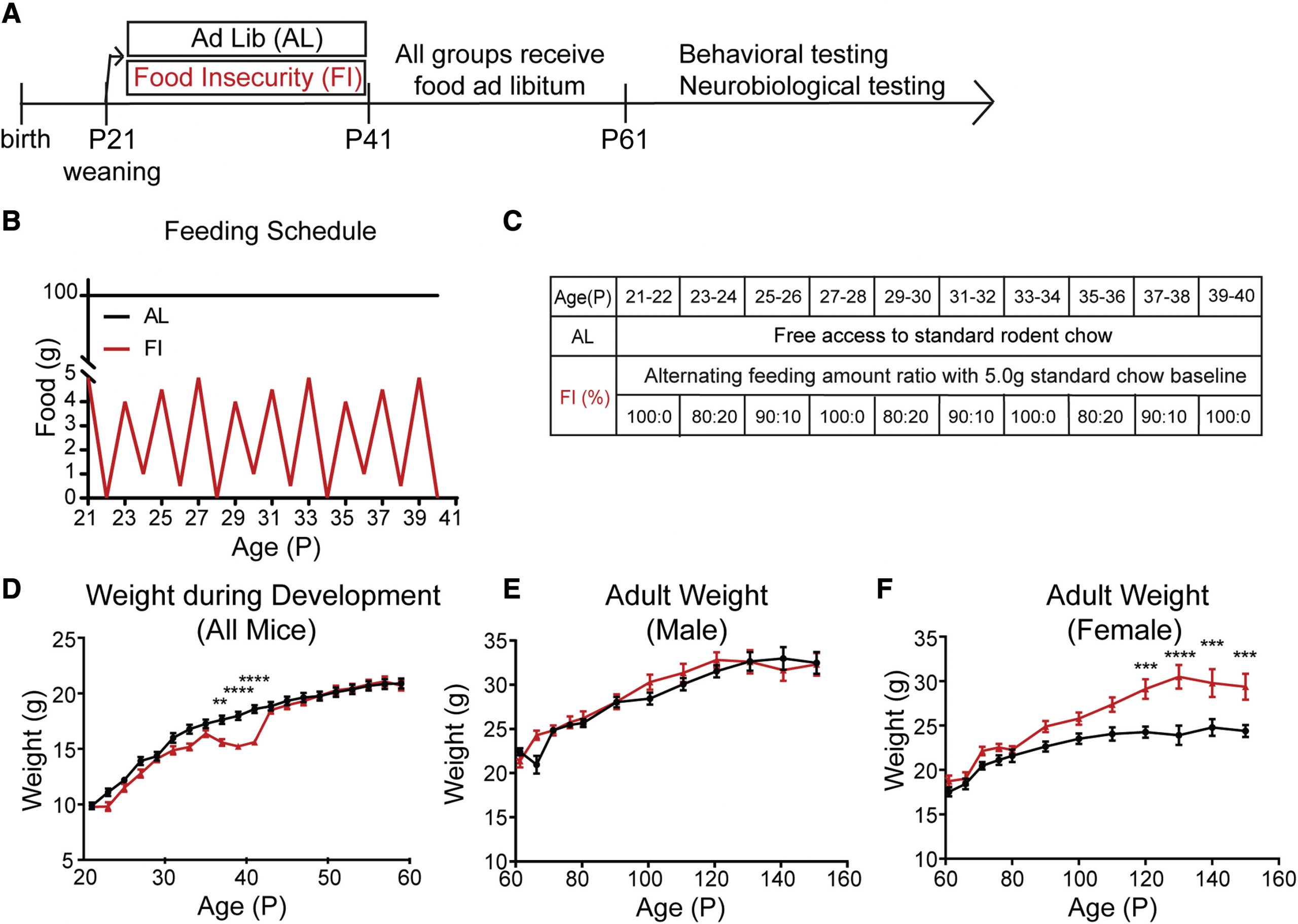
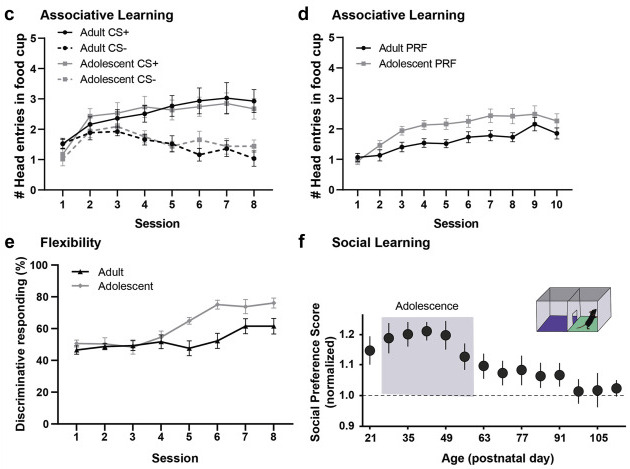
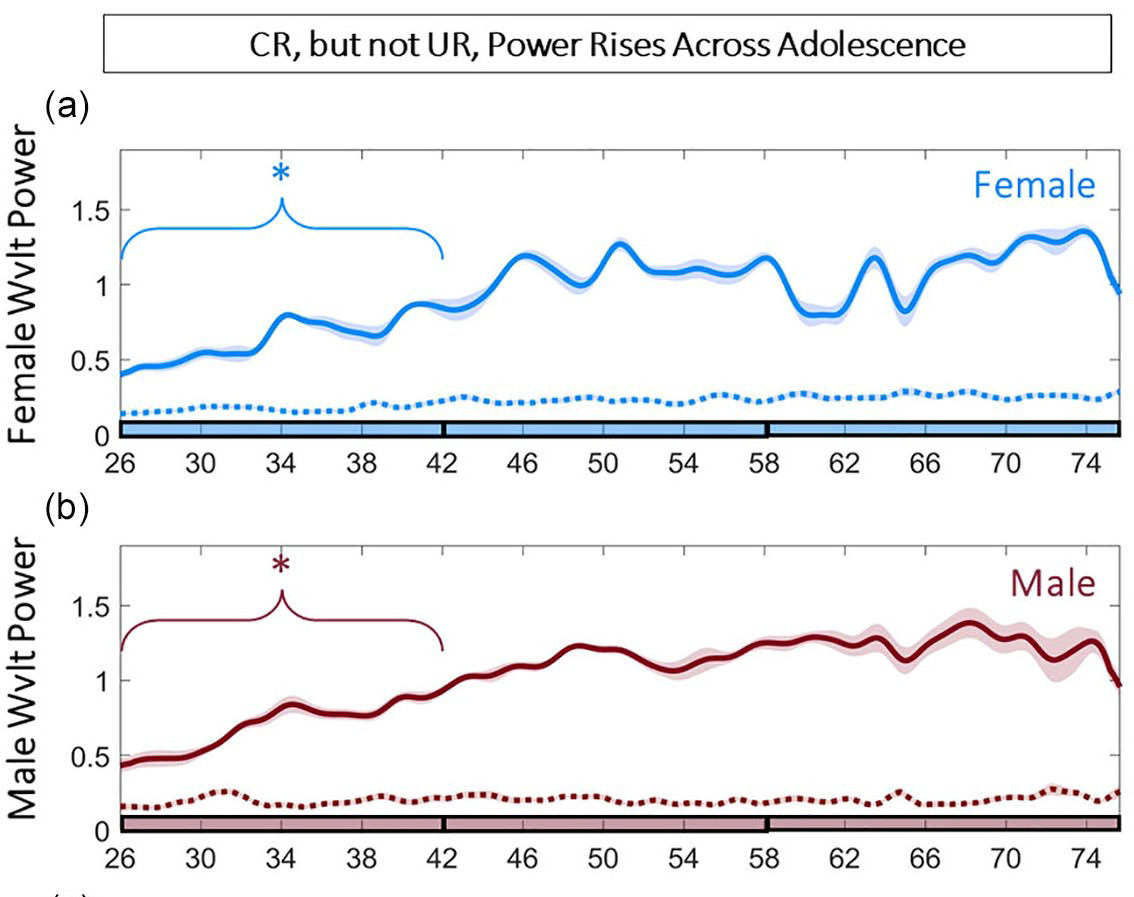
Hidden state inference requires abstract contextual representations in ventral hippocampus
The ability to form and utilize subjective, latent contextual representations to influence decision making is a crucial determinant of everyday life. The hippocampus is widely hypothesized to bind together otherwise abstract combinations of stimuli to represent such latent contexts, and to allow their use to support the process of hidden state inference. Yet, direct evidence for this remains limited. Here we show that the CA1 area of the ventral hippocampus is necessary for mice to perform hidden state inference during a 2-armed bandit task. vCA1 neurons robustly differentiate between the two abstract contexts required for this strategy in a manner similar to the differentiation of spatial locations, despite the contexts being formed only from past probabilistic outcomes. These findings offer insight into how latent contextual information is used to optimize decision-making processes, and emphasize a key role of the hippocampus in hidden state inference.