Prepubertal ovariectomy alters dorsomedial striatum indirect pathway neuron excitability and explore/exploit balance in female mice
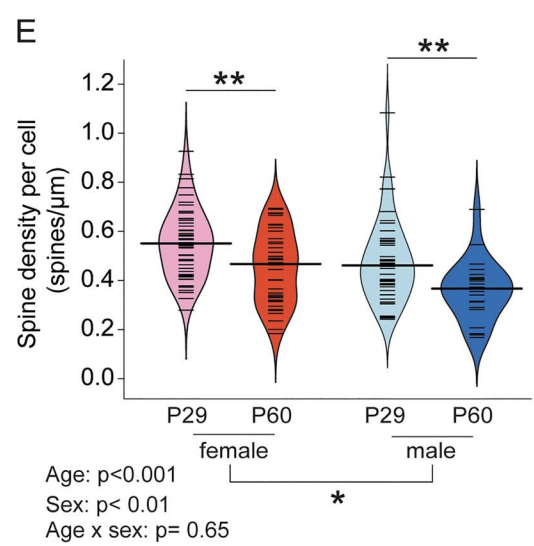
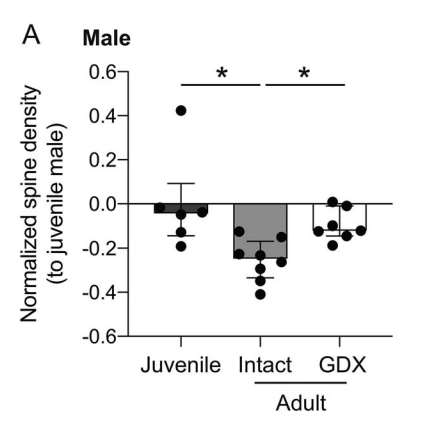
Decision-making circuits are modulated across life stages (e.g. juvenile, adolescent, or adult)—as well as on the shorter timescale of reproductive cycles in females—to meet changing environmental and physiological demands. Ovarian hormonal modulation of relevant neural circuits is a potential mechanism by which behavioral flexibility is regulated in females. Here we examined the influence of prepubertal ovariectomy (pOVX) versus sham surgery on performance in an odor-based multiple choice reversal task. We observed that pOVX females made different types of errors during reversal learning compared to sham surgery controls. Using reinforcement learning models fit to trial-by-trial behavior, we found that pOVX females exhibited lower inverse temperature parameter (β) compared to sham females. These findings suggest that OVX females solve the reversal task using a more exploratory choice policy, whereas sham females use a more exploitative policy prioritizing estimated high value options. To seek a neural correlate of this behavioral difference, we performed whole-cell patch clamp recordings within the dorsomedial striatum (DMS), a region implicated in regulating action selection and explore/exploit choice policy. We found that the intrinsic excitability of dopamine receptor type 2 (D2R) expressing indirect pathway spiny projection neurons (iSPNs) was significantly higher in pOVX females compared to both unmanipulated and sham surgery females. Finally, to test whether mimicking this increase in iSPN excitability could recapitulate the pattern of reversal task behavior observed in pOVX females, we chemogenetically activated DMS D2R(+) neurons within intact female mice. We found that chemogenetic activation increased exploratory choice during reversal, similar to the pattern we observed in pOVX females. Together, these data suggest that pubertal status may influence explore/exploit balance in females via the modulation of iSPN intrinsic excitability within the DMS.
Kristen Delevich, Christopher D. Hall, Linda Wilbrecht, Prepubertal ovariectomy alters dorsomedial striatum indirect pathway neuron excitability and explore/exploit balance in female mice, BioRxiv, https://www.biorxiv.org/content/10.1101/2021.06.01.446609v2, doi: https://doi.org/10.1101/2021.06.01.446609