Transient food insecurity during the juvenile-adolescent period affects adult weight, cognitive flexibility, and dopamine neurobiology
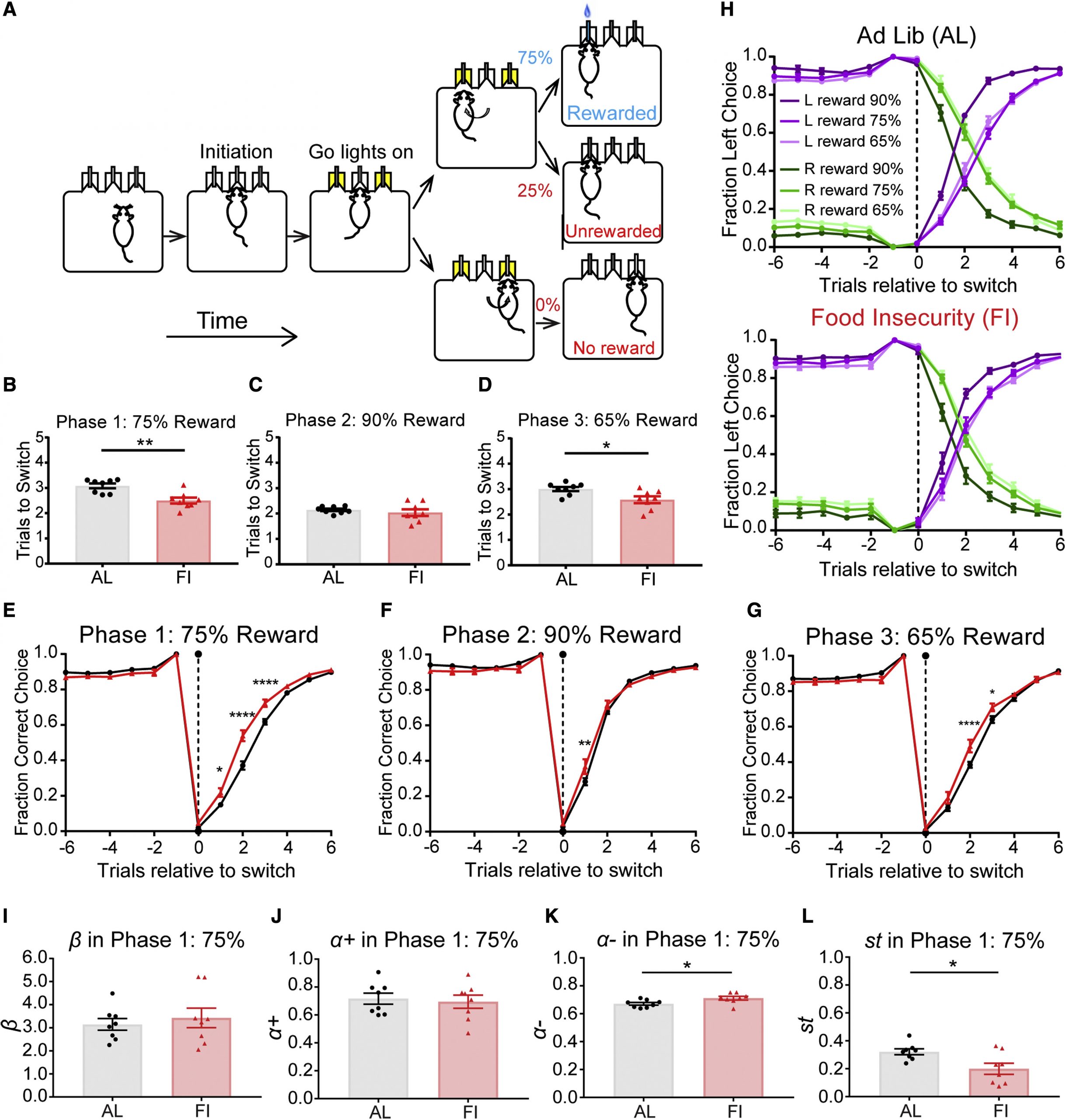
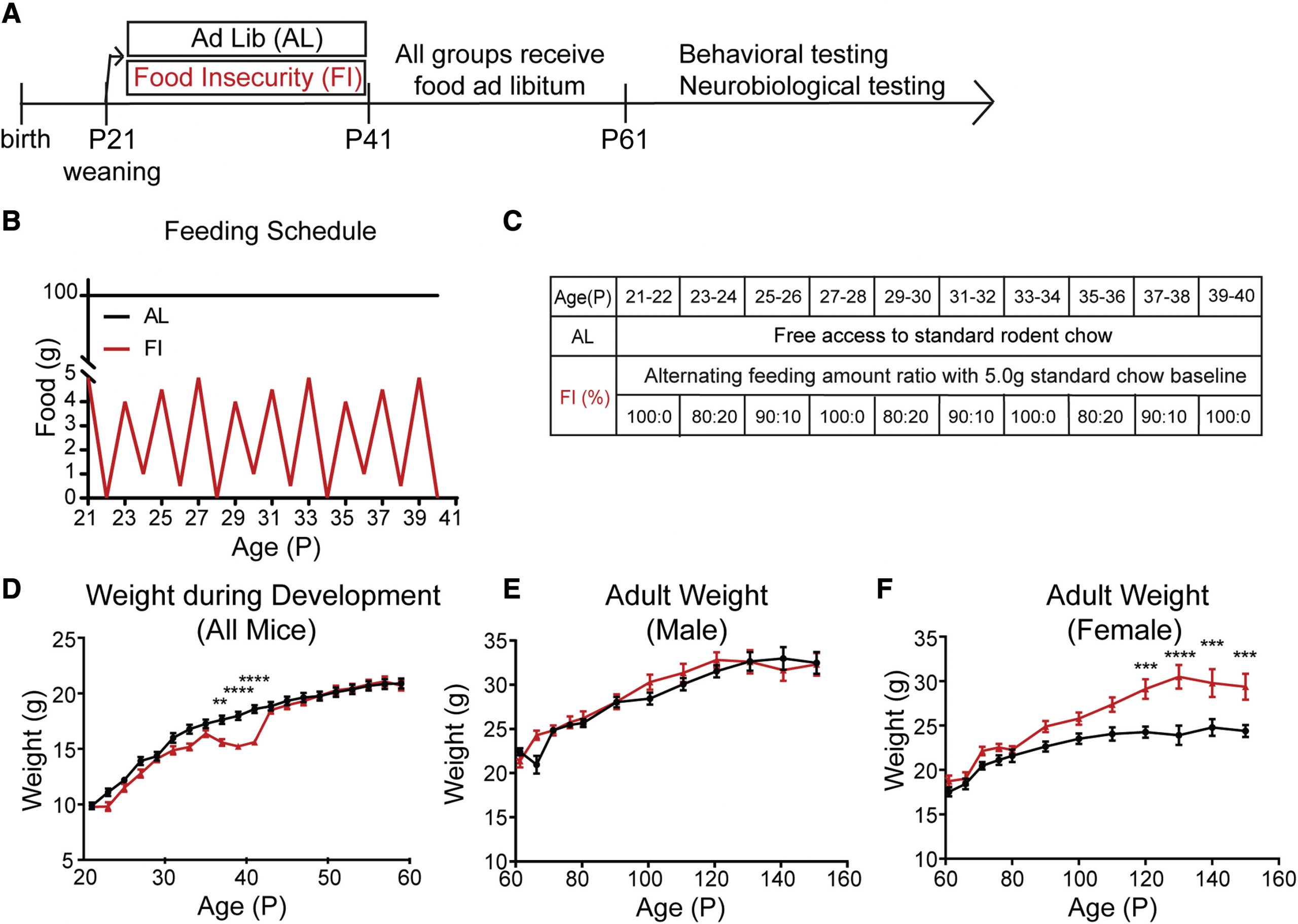
A major challenge for neuroscience, public health, and evolutionary biology is to understand the effects of scarcity and uncertainty on the developing brain. Currently, a significant fraction of children and adolescents worldwide experience insecure access to food. The goal of our work was to test in mice whether the transient experience of insecure versus secure access to food during the juvenile-adolescent period produced lasting differences in learning, decision-making, and the dopamine system in adulthood. We manipulated feeding schedules in mice from postnatal day (P)21 to P40 as food insecure or ad libitum and found that when tested in adulthood (after P60), males with different developmental feeding history showed significant differences in multiple metrics of cognitive flexibility in learning and decision-making. Adult females with different developmental feeding history showed no differences in cognitive flexibility but did show significant differences in adult weight. We next applied reinforcement learning models to these behavioral data. The best fit models suggested that in males, developmental feeding history altered how mice updated their behavior after negative outcomes. This effect was sensitive to task context and reward contingencies. Consistent with these results, in males, we found that the two feeding history groups showed significant differences in the AMPAR/NMDAR ratio of excitatory synapses on nucleus-accumbens-projecting midbrain dopamine neurons and evoked dopamine release in dorsal striatal targets. Together, these data show in a rodent model that transient differences in feeding history in the juvenile-adolescent period can have significant impacts on adult weight, learning, decision-making, and dopamine neurobiology.
Wan Chen Lin, Christine Liu, Polina Kosillo, Lung-Hao Tai, Ezequiel Galarce, Helen S. Bateup, Stephan Lammel, Linda Wilbrecht,
Transient food insecurity during the juvenile-adolescent period affects adult weight, cognitive flexibility, and dopamine neurobiology,
Current Biology, ISSN 0960-9822, (2022) https://doi.org/10.1016/j.cub.2022.06.089 https://www.sciencedirect.com/science/article/pii/S0960982222010946