Tag: Lung-Hao Tai
-
Lung-Hao Tai’s collaborative work with Bo Li lab recently published
Lung-Hao Tai’s collaborative work with the Bo Li lab was recently published as: Marcus Stephenson-Jones, Kai Yu, Sandra Ahrens, Jason M. Tucciarone, Aile N. van Huijstee, Luis A. Mejia, Mario A. Penzo, Lung-Hao Tai, Linda Wilbrecht, Bo Li, A basal ganglia circuit for evaluating action outcomes, Nature, http://dx.doi.org/10.1038/nature19845 (2016).
-

Rule learning enhances structural plasticity of long range axons in frontal cortex
Rules encompass cue-action-outcome associations used to guide decisions and strategies in a specific context. Subregions of the frontal cortex including the orbitofrontal cortex (OFC) and dorsomedial prefrontal cortex (dmPFC) are implicated in rule learning, although changes in structural connectivity underlying rule learning are poorly understood. We imaged OFC axonal projections to dmPFC during training in…
-
Review: Between the Primate and “Reptilian” Brain: Rodent Models Demonstrate the Role of the Corticostriatal Circuits in Decision Making
Decision making can be defined as the flexible integration and transformation of information from the external world into action. Recently, the development of novel genetic tools and new behavioral paradigms has made it attractive to study behavior of all kinds in rodents. By some perspectives, rodents are not an acceptable model for the study of…
-
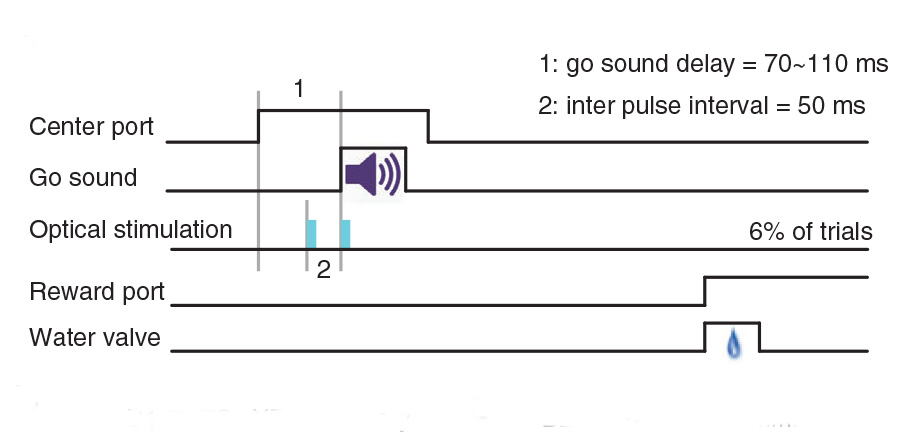
Transient stimulation of distinct subpopulations of striatal neurons mimics changes in action value
In changing environments, animals must adaptively select actions to achieve their goals. In tasks involving goal-directed action selection, striatal neural activity has been shown to represent the value of competing actions. Striatal representations of action value could potentially bias responses toward actions of higher value. However, no study to date has demonstrated the direct effect…
