Tag: Egbert Welker
-
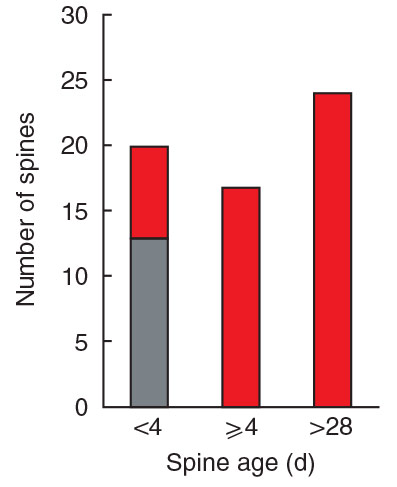
Spine growth precedes synapse formation in the adult neocortex in vivo
Dendritic spines appear and disappear in an experience-dependent manner. Although some new spines have been shown to contain synapses, little is known about the relationship between spine addition and synapse formation, the relative time course of these events, or whether they are coupled to de novo growth of axonal boutons. We imaged dendrites in barrel…
-
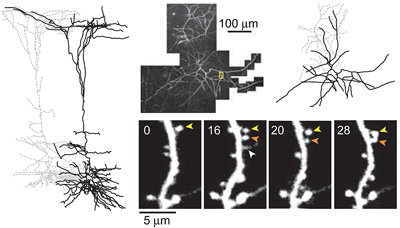
Experience-dependent and cell-type specific spine growth in the neocortex
Functional circuits in the adult neocortex adjust to novel sensory experience, but the underlying synaptic mechanisms remain unknown. Growth and retraction of dendritic spines with synapse formation and elimination could change brain circuits. In the apical tufts of layer 5B (L5B) pyramidal neurons in the mouse barrel cortex, a subset of dendritic spines appear and…
