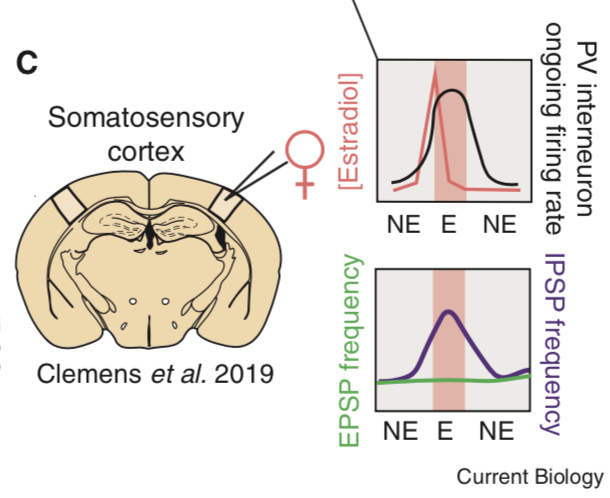
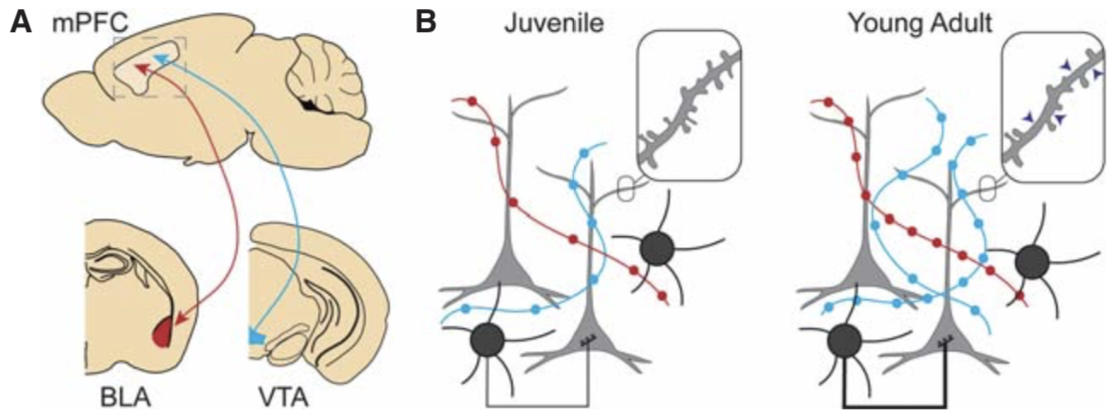
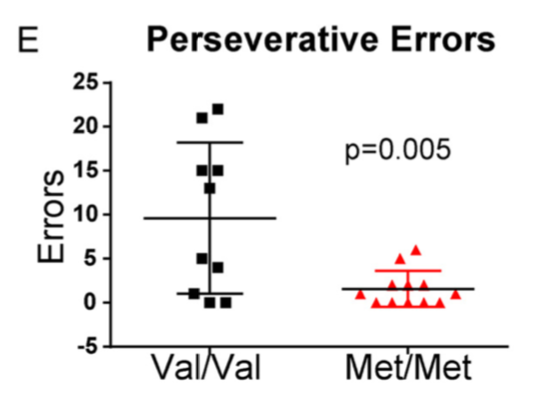
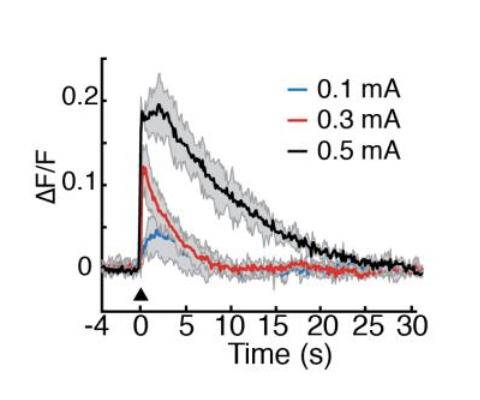
Variation in early life maternal care predicts later long range frontal cortex synapse development in mice
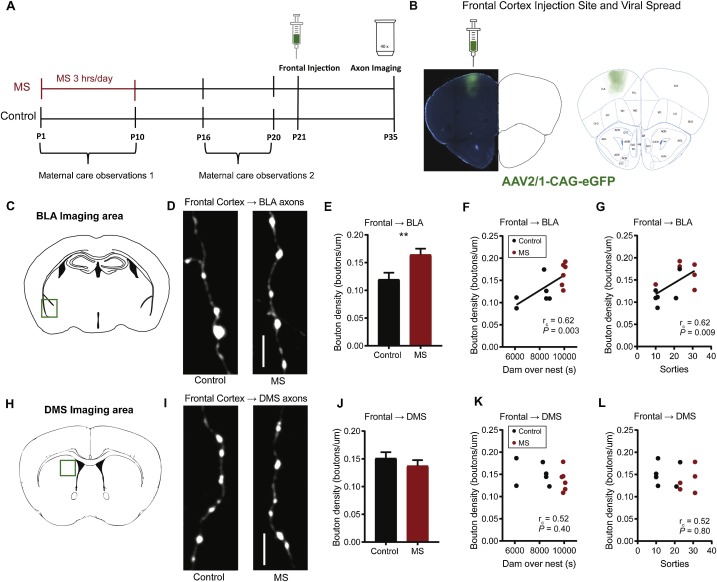
Empirical and theoretical work suggests that early postnatal experience may inform later developing synaptic connectivity to adapt the brain to its environment. We hypothesized that early maternal experience may program the development of synaptic density on long range frontal cortex projections. To test this idea, we used maternal separation (MS) to generate environmental variability and examined how MS affected 1) maternal care and 2) synapse density on virally-labeled long range axons of offspring reared in MS or control conditions. We found that MS and variation in maternal care predicted bouton density on dorsal frontal cortex axons that terminated in the basolateral amygdala (BLA) and dorsomedial striatum (DMS) with more, fragmented care associated with higher density. The effects of maternal care on these distinct axonal projections of the frontal cortex were manifest at different ages. Maternal care measures were correlated with frontal cortex → BLA bouton density at mid-adolescence postnatal (P) day 35 and frontal cortex → DMS bouton density in adulthood (P85). Meanwhile, we found no evidence that MS or maternal care affected bouton density on ascending orbitofrontal cortex (OFC) or BLA axons that terminated in the dorsal frontal cortices. Our data show that variation in early experience can alter development in a circuit-specific and age-dependent manner that may be relevant to early life adversity.
A. Wren Thomas, Kristen Delevich, Irene Chang, Linda Wilbrecht, Variation in early life maternal care predicts later long range frontal cortex synapse development in mice, Developmental Cognitive Neuroscience (2009)
https://doi.org/10.1016/j.dcn.2019.100737, http://www.sciencedirect.com/science/article/pii/S187892931930324X